Challenge
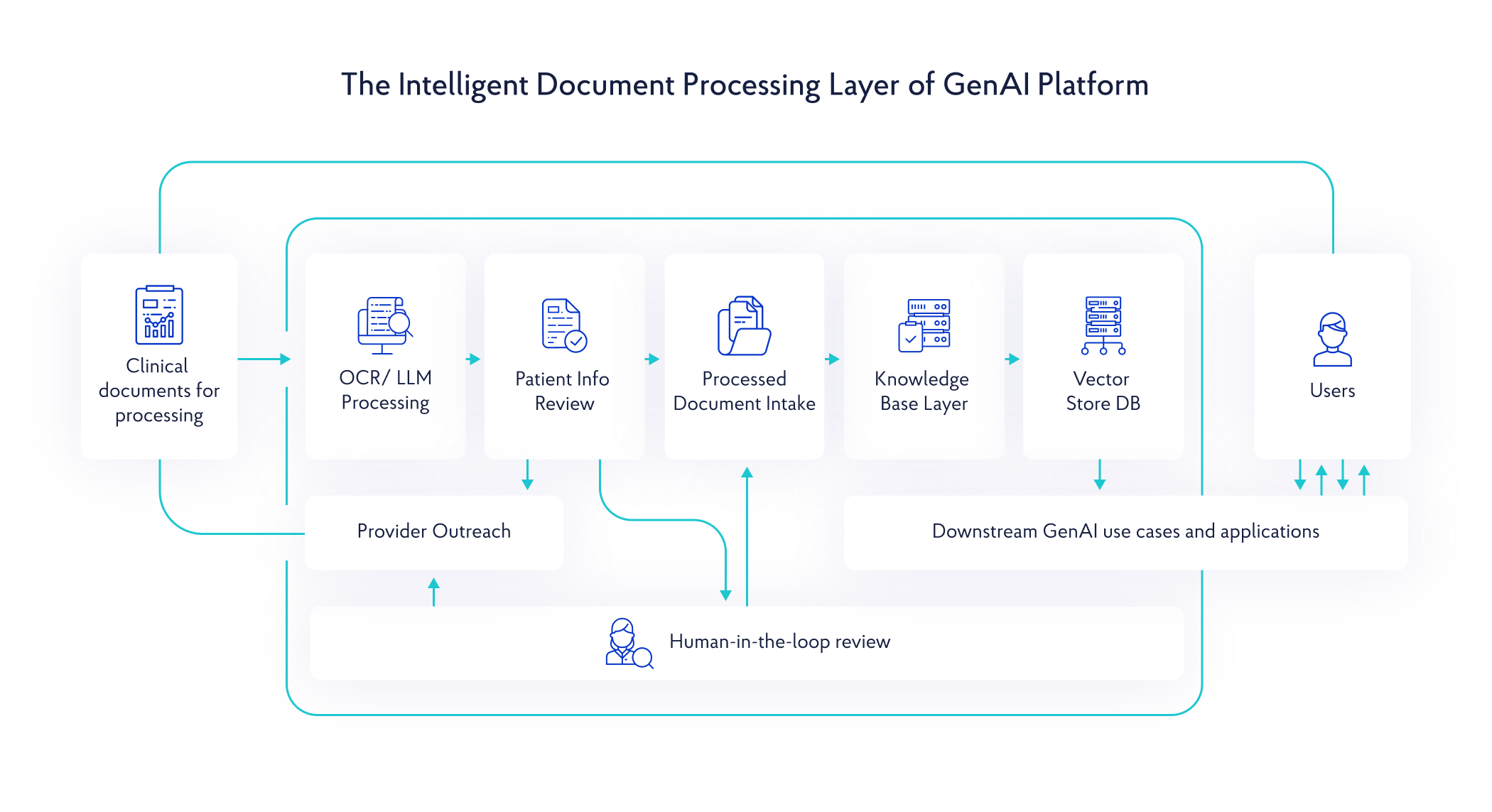
The client managed hundreds of millions of clinical documents – mainly, unstructured clinical notes and pathology reports in inconsistent formats – essential for running tests, supporting providers, and enabling biopharma R&D. Yet the workflows for interpreting these documents were almost entirely manual. Abstractors spent hours per patient reviewing records, extracting key clinical attributes and entering them into “dictionary” spreadsheets, making it impossible to scale operations without growing headcount.
As document volumes continued to increase, workflows became even more fragmented across intake, accessioning, data entry, and billing. Critical clinical information remained locked inside unstructured files, slowing downstream insights for clinicians and limiting the speed at which biopharma partners could build cohorts and prepare validated datasets for clinical trials.
Manual clinical data workflows created an enterprise-wide bottleneck:
- Slower turnaround times
- Rising operational cost
- Data inaccessible for analytics
- Inability to adopt AI for scale
The client envisioned a radical transformation of its clinical data operations, turning millions of unstructured documents into trusted, analytics- & AI-ready data at scale, without compromising accuracy and clinical integrity.

.png)